Share this article and save a life!
The underrepresentation of minority populations in clinical trials is a critical challenge that hinders progress toward equitable healthcare. Despite ongoing advances in medical research, clinical trials often fail to adequately include participants from African American, Latino, and other minority groups. This lack of diversity results in treatments and therapies that may not be as effective or safe for these populations, exacerbating existing disparities in health outcomes.
The Scope of the Problem
According to the FDA, African Americans comprise only 5% of clinical trial participants, while Latinos make up just 1%, despite representing over 30% of the U.S. population combined. This discrepancy underscores a fundamental issue: clinical trials, which serve as the foundation for developing new treatments, often do not reflect the diversity of the real-world patient population. As a result, therapies developed based on narrow, homogenous groups may not account for the unique genetic, environmental, and social factors that affect how minority populations respond to treatment. For example, cancer therapies that show efficacy in predominantly white trials may be less effective or present different safety risks for African American or Latino patients due to variations in tumor biology or drug metabolism.
The implications are profound. The exclusion of minority populations from clinical trials compromises the ability to deliver healthcare that is equitable and effective for all. In some cases, this oversight can lead to life-threatening gaps in care, particularly in fields like oncology, where minority populations often experience worse outcomes and a higher burden of disease.
The Need for Diversity in Clinical Trials
Diversity in clinical trials is not simply a regulatory issue but a scientific necessity. Including participants from diverse racial and ethnic backgrounds allows researchers to gather comprehensive data on how different populations respond to new treatments. This is crucial for ensuring that therapies are safe and effective for everyone, not just a subset of the population.
Genetic, environmental, and social factors can significantly influence treatment outcomes. For example, African American men are 60% more likely to develop prostate cancer than their white counterparts, yet they are underrepresented in clinical studies. Similarly, Latino populations experience disproportionately high rates of diabetes, yet clinical trials for new diabetes treatments often fail to include adequate representation from this group. Without including diverse populations, researchers risk overlooking critical variations in how diseases manifest and treatments perform.
This lack of diversity is particularly concerning in personalized medicine, which seeks to tailor treatments to individual patients based on genetic and environmental factors. Without sufficient data from minority populations, personalized medicine may inadvertently widen the gap in health outcomes, leaving these groups behind.
Challenges to Minority Participation in Clinical Trials
The reasons for underrepresentation in clinical trials are complex and multifaceted. One major barrier is a lack of awareness and education about clinical trials among minority populations. Many individuals from these groups may not be aware that clinical trials are an option for them or may harbor mistrust of the healthcare system due to historical injustices, such as the infamous Tuskegee Syphilis Study. This mistrust and cultural and linguistic barriers can prevent minorities from seeking out or being recruited for clinical trials.
Logistical barriers also play a significant role. Clinical trials are often conducted at large academic medical centers, which may not be easily accessible to individuals from underserved communities. Transportation, childcare, and time off from work can all pose significant obstacles to participation, particularly for individuals from lower socioeconomic backgrounds.
Additionally, there is a lack of concerted efforts by the research and pharmaceutical industries to recruit minority participants actively. Without targeted outreach and engagement, minority populations will continue to be underrepresented in clinical research, perpetuating the cycle of health disparities.
The Role of Federally Qualified Health Centers (FQHCs)
Federally Qualified Health Centers (FQHCs) are uniquely positioned to address this issue and serve as a bridge between minority populations and clinical trials. FQHCs provide comprehensive healthcare services to underserved communities, often in areas with high minority populations. By leveraging their deep community ties and trusted status, FQHCs can increase awareness of clinical trials and facilitate minority participation.
FQHCs are already a vital part of the healthcare safety net, providing care to millions of low-income and minority patients nationwide. Expanding their role to include participation in clinical trials would allow FQHCs to provide cutting-edge treatments to their patients and contribute to developing therapies that are more representative of the population. FQHCs have the infrastructure and trust within their communities to conduct outreach, educate patients about the importance of clinical trials, and address concerns around participation.
Moreover, many FQHCs serve as a clinical extension of academic medical centers or other research institutions, making them well-suited to integrate clinical trials into their care models. By doing so, FQHCs can help ensure that minority populations have access to the latest medical advancements while contributing to a more inclusive body of research.
How Oatmeal Health Can Support FQHCs in Offering Clinical Trials
Oatmeal Health is uniquely positioned to support FQHCs in participating in clinical trials, allowing these centers to offer advanced treatments to their patients while contributing to more diverse and inclusive research.
By placing our clinical nurse practitioners and patient navigators directly at the FQHC, as well as offering remote services, we help integrate clinical trials into their operations. This approach enables resource-constrained centers to screen more patients for cancer and creates a new revenue stream previously unavailable to them.
One of the key ways Oatmeal Health can support FQHCs is through advanced AI technology. By employing AI-driven tools, Oatmeal Health can help FQHCs identify patients who may be eligible for clinical trials based on their medical history, genetics, and other relevant factors. This not only streamlines the process of patient recruitment but also ensures that the right patients are matched with the right trials, improving outcomes for both patients, Oatmeal’s clinical staff that works inside the FQHC, and researchers.
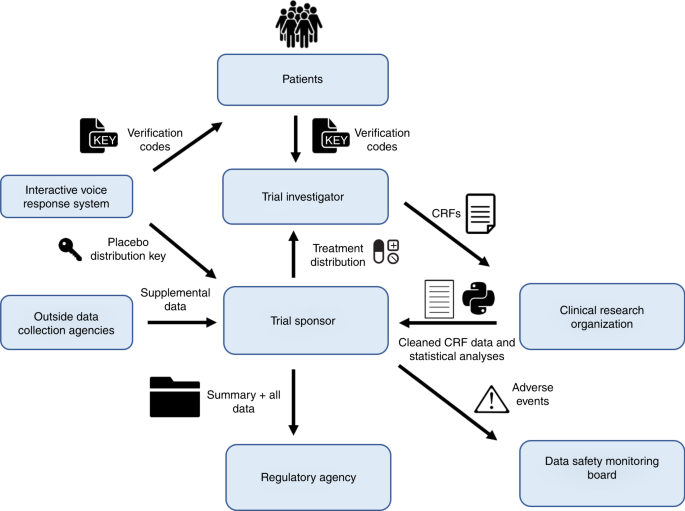
Furthermore, Oatmeal Health can assist FQHCs in navigating the regulatory and logistical complexities of conducting clinical trials. From managing trial protocols to ensuring compliance with ethical standards, Oatmeal Health’s experience in the clinical trial space can help FQHCs overcome the barriers that may otherwise prevent them from offering these opportunities to their patients.
Ultimately, Oatmeal Health’s partnership with FQHCs represents a powerful opportunity to address the underrepresentation of minority populations in clinical trials. By providing FQHCs with the tools and support they need to offer clinical trials to their patients, Oatmeal Health can help ensure that new therapies are developed with all populations in mind, reducing health disparities and advancing health equity.
The Importance of Inclusive Data in Oncology Research
In fields like oncology, where precision medicine is becoming increasingly important, the need for inclusive data is especially urgent. Cancer affects minority populations in unique ways, and therapies that do not account for this diversity may leave these groups behind. For instance, African American men have a higher risk of developing and dying from prostate cancer, yet they are often underrepresented in clinical trials for new cancer treatments.
By prioritizing the inclusion of underrepresented populations in oncology research, we can develop more effective cancer therapies that serve all demographics. This not only ensures that minority populations receive the best possible care but also advances the field of oncology by providing researchers with the data they need to identify variations in drug responses and improve outcomes for all patients.
Conclusion
The underrepresentation of minority populations in clinical trials is a significant barrier to achieving equitable healthcare. By failing to include diverse participants, clinical research risks leaving behind populations that are already disproportionately affected by chronic diseases like cancer, diabetes, and heart disease.
Federally Qualified Health Centers (FQHCs) are uniquely positioned to address this issue by offering clinical trials to their patients. With the support of organizations like Oatmeal Health, which can provide AI-driven tools and expertise in clinical trial management, FQHCs can help bridge the gap in minority participation and contribute to the development of more effective and inclusive treatments.
As medicine evolves toward personalized care, including diverse populations in clinical research will be critical for driving innovation and improving patient outcomes. By embracing this challenge, we can move closer to a future where healthcare is truly equitable for all.
Share this article and save a life!
Author:

Jonathan is a seasoned executive with a proven track record in founding and scaling digital health and technology companies. He co-founded Oatmeal Health, a tech-enabled Cancer Screening as a Service for Underrepresented patients of FQHCs and health plans, starting with lung cancer. With a strong background in engineering, partnerships, and product development, Jonathan is recognized as a leader in the industry.
Govette has dedicated his professional life to enhancing the well-being of marginalized populations. To achieve this, he has established frameworks for initiatives aimed at promoting health equity among underprivileged communities.